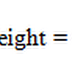07 Equivalent Weight
STP or NTP
Pressure = 760 mmHg = 1 atm
T = 273K = 0°c
100ml = 1000cc = 1 Litre
At STP 1 ml or 1 cc of hydrogen = 0.000089 gm
Atomic Weight = Equivalent Weight × Valency
In oxide formation method:
In Hydrogen Displacement Method:
In Chlorine Formation Method:
Q. 0.212 gram of magnesium when dissolve in hydrochloric acid and the volume of hydrogen collected over water at 16°c and 750mmHg pressure was 213.5ml. Calculate the equivalent weight of metal.
Solution:
Weight of metal = 0.212 gm
For Hydrogen,
Temperature (T1) =16°c = (16 + 273)K = 289 K
Pressure (P1) = 750 mmHg
Volume(V1) = 213.5ml
At STP, Temperature (T2) = 273K
Pressure (P2) = 760mmHg
Volume (V2) = ?
By using combined gas equation,
Again, at STP or NTP
1 ml or 1 cc of hydrogen = 0.000089gm
199.026ml of hydrogen = 0.000089×199.026gm
= 0.0177gm
Pressure = 760 mmHg = 1 atm
T = 273K = 0°c
100ml = 1000cc = 1 Litre
At STP 1 ml or 1 cc of hydrogen = 0.000089 gm
Atomic Weight = Equivalent Weight × Valency
In oxide formation method:
In Hydrogen Displacement Method:
In Chlorine Formation Method:
Q. 0.212 gram of magnesium when dissolve in hydrochloric acid and the volume of hydrogen collected over water at 16°c and 750mmHg pressure was 213.5ml. Calculate the equivalent weight of metal.
Solution:
Weight of metal = 0.212 gm
For Hydrogen,
Temperature (T1) =16°c = (16 + 273)K = 289 K
Pressure (P1) = 750 mmHg
Volume(V1) = 213.5ml
At STP, Temperature (T2) = 273K
Pressure (P2) = 760mmHg
Volume (V2) = ?
By using combined gas equation,
1 ml or 1 cc of hydrogen = 0.000089gm
199.026ml of hydrogen = 0.000089×199.026gm
= 0.0177gm