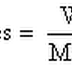08 Molecular Weight
Molecular Weight is the number which shows how many times a molecule of a substance is heavier than 1/12th part of an atom of C12 isotope. In other words molecular weight is the sum of atomic weight of all atoms present in a molecule of a substance.
eg. Mol. wt. of CaCO3 =1×40 + 1×12 + 3×16 = 100
Gram Molecular Weight
When molecular weight of a substance is expressed in gram, then it is called gram molecular weight. One gram molecular weight equals to one mole and its contains 6.023×1023 molecules.
For Eg.
Molecular Weight of CaCO3 = 100
100gm of CaCO3 = 1 gram molecular weight of CaCO3
= 1 Mole of CaCO3
= 6.023×1023 molecules of CaCO3
Q. Calculate the number of moles and number of molecules present in 80gm of CaCO3.
Solution:
Molecular Weight of CaCO3 = 100
100gm of CaCO3 = 1 Mole of CaCO3
eg. Mol. wt. of CaCO3 =1×40 + 1×12 + 3×16 = 100
Gram Molecular Weight
When molecular weight of a substance is expressed in gram, then it is called gram molecular weight. One gram molecular weight equals to one mole and its contains 6.023×1023 molecules.
For Eg.
Molecular Weight of CaCO3 = 100
100gm of CaCO3 = 1 gram molecular weight of CaCO3
= 1 Mole of CaCO3
= 6.023×1023 molecules of CaCO3
Q. Calculate the number of moles and number of molecules present in 80gm of CaCO3.
Solution:
Molecular Weight of CaCO3 = 100
100gm of CaCO3 = 1 Mole of CaCO3
Q. Calculate the number of atoms of Ca, C and O present in 150gm of CaCO3.
Solution:
Molecular weight of CaCO3 = 100
Weight of CaCO3 = 150gram
Number of Moles = ?
Number of Molecules = ?
Number of atoms of Ca, C and O = ?
Number of molecules = Number of moles × 6.023×1023
= 1.5×6.023×1023
= 9.0345×1023
1 molecule of CaCO3 contains 1 atom of Ca.
9.0345×1023 molecule of CaCO3 contains 9.0345×1023 atoms of Ca.
1 molecule of CaCO3 contains 1 atom of C.
9.0345×1023 molecule of CaCO3 contains 9.0345×1023 atoms of C.
1 molecule of CaCO3 contains 3 atoms of Oxyen.
9.0345×1023 molecule of CaCO3 contains 3×9.0345×1023 atoms of C.
= 2.7103×1023 atoms of C.
Q. Calculate the number of moles and number of atoms of oxygen present in 80gm of oxygen.
Solution:
Molecular Weight of Oxygen = 32
Weight of Oxygen = 80gm
Number of molecules = 2.5 × 6.023×1023
= 1.5058×1024
One molecule of oxygen contains 2 atoms of oxyegn.
1.5058×1024 molecules of oxygen contains 2×1.5058×1024
= 3.01×1024 atoms of oxygen